How Do Animals Get Carbon Into Their Bodies
There are a few types of atoms that tin be a part of a establish one day, an brute the adjacent day, so travel downstream as a function of a river'southward water the following day. These atoms can be a part of both living things like plants and animals, likewise as non-living things like h2o, air, and even rocks. The same atoms are recycled over and over in different parts of the Earth. This type of wheel of atoms between living and non-living things is known as a biogeochemical cycle.
All of the atoms that are building blocks of living things are a office of biogeochemical cycles. The most common of these are the carbon and nitrogen cycles.
Tiny atoms of carbon and nitrogen are able to move effectually the planet through these cycles. For example, an cantlet of carbon is absorbed from the air into the ocean h2o where it is used past little floating plankton doing photosynthesis to get the nutrition they need. There is the possibility that this little carbon atom becomes role of the plankton's skeleton, or a part of the skeleton of the larger animal that eats it, and so role of a sedimentary rock when the living things die and but bones are left backside. Carbon that is a function of rocks and fossil fuels like oil, coal, and natural gas may be held away from the rest of the carbon wheel for a long time. These long-term storage places are called "sinks". When fossil fuels are burned, carbon that had been underground is sent into the air as carbon dioxide, a greenhouse gas.
Recently, people accept been causing these biogeochemical cycles to change. When nosotros cut down forests, make more factories, and bulldoze more cars that burn fossil fuels, the manner that carbon and nitrogen movement around the Earth changes. These changes add together more greenhouse gases in our atmosphere and this causes climatic change.
The Carbon Cycle
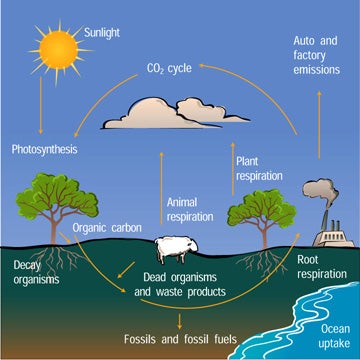
The element carbon is a role of seawater, the atmosphere, rocks such equally limestone and coal, soils, as well every bit all living things. On our dynamic planet, carbon is able to move from 1 of these realms to another as a part of the carbon cycle.
- Carbon moves from the atmosphere to plants. In the atmosphere, carbon is fastened to oxygen in a gas called carbon dioxide (CO2). Through the process of photosynthesis, carbon dioxide is pulled from the air to produce food fabricated from carbon for plant growth.
- Carbon moves from plants to animals. Through food chains, the carbon that is in plants moves to the animals that eat them. Animals that eat other animals get the carbon from their food too.
- Carbon moves from plants and animals to soils. When plants and animals die, their bodies, wood and leaves decays bringing the carbon into the ground. Some is buried and will go fossil fuels in millions and millions of years.
- Carbon moves from living things to the atmosphere. Each fourth dimension you exhale, you are releasing carbon dioxide gas (CO2) into the atmosphere. Animals and plants demand to get rid of carbon dioxide gas through a process chosen respiration.
- Carbon moves from fossil fuels to the atmosphere when fuels are burned. When humans burn fossil fuels to ability factories, ability plants, cars and trucks, most of the carbon quickly enters the atmosphere as carbon dioxide gas. Each yr, five and a one-half billion tons of carbon is released past burning fossil fuels. Of this massive amount, 3.3 billion tons stays in the atmosphere. Well-nigh of the residue becomes dissolved in seawater.
- Carbon moves from the atmosphere to the oceans. The oceans, and other bodies of water, blot some carbon from the atmosphere. The carbon is dissolved into the water.
Carbon dioxide is a greenhouse gas and traps oestrus in the atmosphere. Without it and other greenhouse gases, Earth would exist a frozen earth. Simply since the showtime of the Industrial Revolution about 150 years ago humans have burned so much fuel and released so much carbon dioxide into the air that global climate has risen over 1 degree Fahrenheit. The atmosphere has not held this much carbon for at least 420,000 years according to data from ice cores. The recent increase in amounts of greenhouse gases such as carbon dioxide is having a significant bear upon on the warming of our planet.
Carbon moves through our planet over longer fourth dimension scales as well. For example, over millions of years weathering of rocks on country can add carbon to surface water which eventually runs off to the body of water. Over long time scales, carbon is removed from seawater when the shells and bones of marine animals and plankton collect on the sea floor. These shells and bones are made of limestone, which contains carbon. When they are deposited on the bounding main flooring, carbon is stored from the remainder of the carbon cycle for some amount of time. The amount of limestone deposited in the ocean depends somewhat on the corporeality of warm, tropical, shallow oceans on the planet because this is where prolific limestone-producing organisms such as corals live. The carbon can be released dorsum to the atmosphere if the limestone melts or is metamorphosed in a subduction zone.
The Nitrogen Cycle
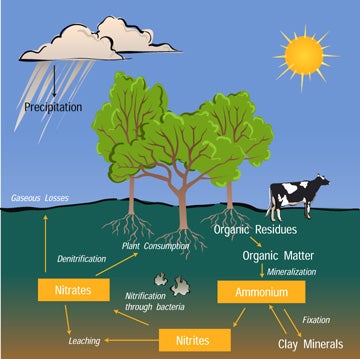
Nitrogen is an element that is found in both the living portion of our planet and the inorganic parts of the Earth organisation. Nitrogen moves slowly through the cycle and is stored in reservoirs such every bit the atmosphere, living organisms, soils, and oceans forth the manner.
Most of the nitrogen on Earth is in the atmosphere. Approximately eighty% of the molecules in Earth's atmosphere are made of two nitrogen atoms bonded together (Northward2). All plants and animals demand nitrogen to make amino acids, proteins and DNA, but the nitrogen in the temper is not in a form that they tin can apply. The molecules of nitrogen in the atmosphere tin can become usable for living things when they are broken apart during lightning strikes or fires, by certain types of leaner, or past leaner associated with legume plants. Other plants become the nitrogen they demand from the soils or water in which they live mostly in the course of inorganic nitrate (NO3-). Nitrogen is a limiting factor for plant growth. Animals become the nitrogen they need past consuming plants or other animals that contain organic molecules composed partially of nitrogen. When organisms die, their bodies decompose bringing the nitrogen into soil on land or into the oceans. Every bit dead plants and animals decompose, nitrogen is converted into inorganic forms such equally ammonium salts (NH4+) by a process called mineralization. The ammonium salts are captivated onto clay in the soil so chemically contradistinct by bacteria into nitrite (NO2-) and then nitrate (NO3-). Nitrate is the form commonly used past plants. It is hands dissolved in water and leached from the soil system. Dissolved nitrate tin be returned to the temper by certain leaner through a process chosen denitrification.
Certain actions of humans are causing changes to the nitrogen cycle and the corporeality of nitrogen that is stored in reservoirs. The use of nitrogen-rich fertilizers tin can cause nutrient loading in nearby waterways as nitrates from the fertilizer launder into streams and ponds. The increased nitrate levels cause plants to grow rapidly until they apply upwards the nitrate supply and die. The number of herbivores will increase when the plant supply increases and then the herbivores are left without a food source when the plants die. In this way, changes in food supply volition affect the entire food chain. Additionally, humans are altering the nitrogen cycle by burning fossil fuels and forests, which releases various solid forms of nitrogen. Farming also affects the nitrogen cycle. The waste product associated with livestock farming releases a large amount of nitrogen into soil and water. In the same way, sewage waste matter adds nitrogen to soils and h2o.
Nitrogen and Air Pollution

An unsightly brume of smog, visible from NCAR'due south Mesa Laboratory, rests over Boulder Valley.
UCAR
Nitric oxide (NO) and nitrogen dioxide (NO2) are together known equally nitrogen oxides. These nitrogen oxides contribute to the problem of air pollution, playing roles in the formation of both smog and acid rain. They are released into Earth'southward atmosphere by both natural and human-generated sources.
Nitric oxide is a colorless, flammable gas with a slight odor. Nitrogen dioxide is a deep red-orange gas that is poisonous but not flammable. It, along with aerosols, is responsible for the ruby-brown color of smog. At high concentrations, information technology is highly toxic and can cause serious lung damage. Nitrogen dioxide is a stiff oxidizing amanuensis, and is thus very reactive with other compounds.
Scientists estimate that between 20 and xc million tons of nitrogen oxides in produced naturally each year from sources such as volcanoes, oceans, biological decay, and lightning strikes. Homo activities add some other 24 meg tons of nitrogen oxides to our atmosphere annually.
Both NO and NO2 are formed during loftier-temperature combustion in the atmosphere, when oxygen combines with nitrogen. The exhaust gases of cars and trucks are major sources of nitrogen oxides, as are the emissions from electrical power generation plants. Automobile exhaust has more NO than NO2, just in one case the NO is released into the atmosphere it quickly combines with oxygen in the air to form NO2.
Nitrogen oxides are at least partially responsible for several types of air pollution. Nitrogen dioxide lends its colour to the scarlet-brown haze we call smog. Photodissociation of nitrogen dioxide by sunlight produces nitric oxide and ozone in the troposphere, which is another component of smog. A series of chemical reactions transform Volatile Organic Compounds (VOCs) into substances that combine with nitrogen dioxide to produce PAN (Peroxyacytyl nitrate), withal another chemical element in smog. Nitrogen dioxide in the air also reacts with water vapor to course nitric acrid, 1 of the types of acid in acrid rain. Nitric oxide concentration in unpolluted air is effectually 0.01 ppm. In smog, the concentration rises 20-fold to about 0.2 ppm.
Although nitrogen oxides have gained dubious distinction as pollutants, they are likewise used beneficially in some industrial processes. Nitric oxide is manufactured on a large calibration, and is subsequently used to make nitric acid (HNO3). To create nitric oxide for industrial uses, chemists combine ammonia (NH3) with oxygen (O2), releasing h2o (HiiO) equally a byproduct. Nitrogen compounds derived from nitric acid are used to create chemical fertilizers, explosives, and other useful substances.
© 2011 NESTA with modifications by UCAR
Source: https://scied.ucar.edu/learning-zone/earth-system/biogeochemical-cycles
Posted by: hambybuir1998.blogspot.com

0 Response to "How Do Animals Get Carbon Into Their Bodies"
Post a Comment